Kids aren't just littler adults – here's why they need their own clinical trials for a COVID-19 vaccine
- Written by Judy Martin, Professor of Pediatrics, University of Pittsburgh
Now that two-thirds of all adults[1] in the United States have received at least one dose of a SARS-CoV-2 vaccine as of mid-July 2021, life seems to be returning to some semblance of pre-pandemic times. People are again traveling, eating in restaurants with friends, attending in-person gatherings and flocking to movie theaters[2] and Major League baseball games[3].
Yet for parents of children under the age of 12, who are not yet eligible for COVID-19 vaccines, there is still no collective sigh of relief. Many parents have concerns about the upcoming school year[4] and the uncertainty surrounding the delta variant[5].
Clinical research studies of the mRNA-based vaccines for children under 12 are ongoing, and authorization of a vaccine for this younger age group is still at least several months away. These trials are necessary because children have important differences in physiology and responses to vaccines[6] from those of adults. Conducting separate studies in children under age 12 is a vital step toward ending the pandemic.
As a specialist in pediatric infectious diseases[7], I have been conducting research on common infections in children and related vaccines for over 20 years. Here at the University of Pittsburgh, our Pittsburgh Vaccine Trials Unit[8] has carried out both adult and pediatric clinical trials for vaccines to fight COVID-19.
Ours was one of two COVID-19 vaccine clinical research trial sites in the Pittsburgh area and one of more than 100 sites across the U.S. that have participated in this effort through the COVID-19 Prevention Network[9], which was formed by the National Institutes of Health to combat the spread of the coronavirus. Our team is about to begin the next phase of trials with the 6-11 year-old age group, which relies on volunteer participants.
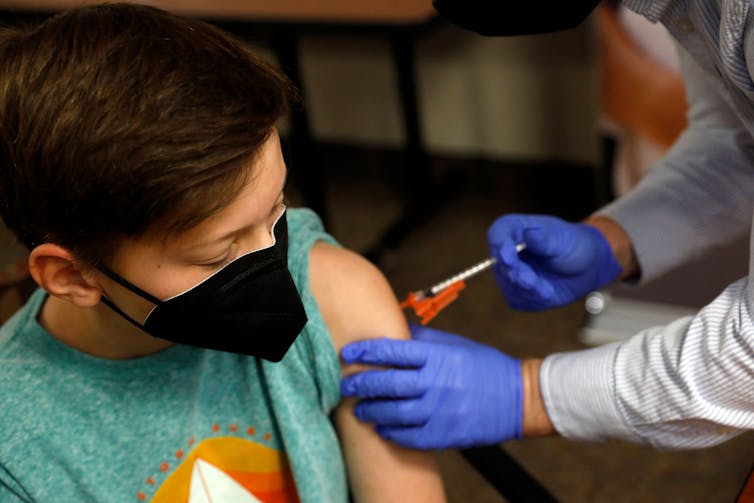 The FDA authorized the use of the Pfizer COVID-19 vaccine for 12-to-15-year-olds in May.
Jeff Kowalsky/AFP via Getty Images[10]
The FDA authorized the use of the Pfizer COVID-19 vaccine for 12-to-15-year-olds in May.
Jeff Kowalsky/AFP via Getty Images[10]
Testing a vaccine for safety and efficacy
Vaccines work by tricking the body’s immune system into making proteins, called antibodies, that fight disease – but without giving a person the disease.
Before a vaccine can be approved for use in the general public, it usually goes through clinical safety trials that can take anywhere from 2 to 15 years[11]. The U.S. goverment’s Operation Warp Speed[12] accelerated this process in an unprecedented way, largely because it invested US$18 billion up front to help create lab spaces, build infrastructure, make research investments and pre-purchase vaccines. In December 2020, health care workers in the U.S. began receiving[13] the first COVID-19 vaccines authorized for adults.
Vaccine studies begin with experiments in the laboratory, where candidate vaccines are developed and tested in animals. After pharmaceutical companies and government labs perform initial testing on vaccine candidates, they then turn to research groups throughout the country and world to run several phases of clinical trials[14] in people.
In phase 1 trials, the primary goal is to establish the safety of the vaccine in humans. During phase 2, researchers continue to evaluate the safety of the vaccine, but with an eye to determining the exact dosage needed to achieve the necessary immune response to confer protection. Once a vaccine candidate enters phase 3 trials, the primary goal is to study how well people are protected from the infection or disease, while continuing to assess safety and monitor for potential side effects.
Once clinical trials are complete, vaccines must still undergo a rigorous evaluation process[15] through the U.S. Food and Drug Administration, the regulatory body that oversees vaccine safety and effectiveness.
After tens[16] of thousands[17] of adults[18] participated in phase 3 clinical research studies of COVID-19 vaccines over several months in 2020 and early 2021, the U.S. now has three vaccines[19] authorized for emergency use[20] by the FDA for people 18 years of age and older and one vaccine, Pfizer, authorized for use in children age 12 and older.
How kids’ bodies differ from grown-ups’
Children are not just littler grownups; their bodies differ from adults’ in important ways.
Their brains are developing rapidly[21], and their immune systems have important differences too, particularly in toddlers and babies. For the first few months of life, infants’ immune systems still possess the antibodies they received from their mothers[22] across the placenta during late pregnancy. This changes how newborns respond to pathogens and makes them less able to mount an immune response to some vaccines. Young children’s bodies gradually ramp up their own immune systems as their protection from mom wears off.
So vaccines often need to be tailored specifically for young children. For instance, the Pneumococcal vaccine that prevents infections[23] like pneumonia in adults is made from sugar molecules called polysaccharides, which coat the outside of pneumococcal bacteria. But infants can’t mount an effective immune response to these sugar molecules. So researchers had to develop a unique version of the vaccine[24] for babies.
 Masks and temperature checks will likely be the norm for many U.S. school-going kids until a COVID-19 vaccine is authorized for children under age 12.
NurPhoto/Getty Images[25]
Masks and temperature checks will likely be the norm for many U.S. school-going kids until a COVID-19 vaccine is authorized for children under age 12.
NurPhoto/Getty Images[25]
Even when a vaccine for adults is proven safe in children, there can be important differences in how their bodies respond to it. The vaccine dose that works best in adults might cause a high fever in children, for instance. So one key goal of the COVID-19 vaccine clinical trials in children will be to determine the optimal dosage for each age group.
Researchers need to be on alert for side effects that might only occur in youngsters and didn’t appear during vaccine tests on adults. Safety is critical and each study has many layers of safety mechanisms in place to ensure that researchers like us proceed cautiously and evaluate all of the data and information at every step along the way.
For example, trial participants keep daily diaries and report any side effects or changes. Vaccine clinical trials include frequent safety checks with participants, and unusual reactions are reported immediately to the study sponsor so that any problems can be identified quickly. Researchers also adhere to strict “pause” rules if a serious safety concern arises.
Clinical trials for kids
After setting up a new clinical trials space and gathering all the staff and necessary equipment, the Trials Unit here at the University of Pittsburgh was ready to host phase 3 clinical trials with volunteer participants.
Beginning in August 2020 and into the fall, we ran phase 3 adult clinical trials for both the Moderna and Johnson & Johnson vaccines. We recently enrolled kids ages 6-11 as well as 6 to 24 months of age in phase 2 of the pediatric Moderna trials, focusing on whether the vaccine is safe to use in these kids and at what dosages.
Our site is now set to move to phase 3 of the pediatric trials, currently slated to begin in mid-August for children age 6-11, throughout the U.S. and Canada. This final stage of the clinical trials will determine how well the vaccine really works to keep kids from getting COVID-19. We expect early results of these studies by this fall, after which they will be reviewed by the FDA.
[Like what you’ve read? Want more? Sign up for The Conversation’s daily newsletter[26].]
The FDA said on July 15 that emergency authorization for vaccines for children under 12[27] is likely to come by early to mid-winter.
The vital role of volunteers in ending the pandemic
Volunteering for a research study is not for everyone.
When a family volunteers to enroll in a vaccine study, our research team has an in-depth discussion with them about the requirements, as well as the potential risks and benefits. We try to answer all of their questions so that they can decide if a study is a good fit for them. Ultimately, parents are trying to make a decision that is in the best interest of their child.
Often we hear from our volunteers that they wanted to help bring the pandemic to an end or felt it was their personal responsibility to help others. Their willingness to participate is crucial to finding a safe and effective vaccine that will hopefully help end the pandemic and to help parents – and kids – return to the freedoms of pre-pandemic life.
References
- ^ two-thirds of all adults (covid.cdc.gov)
- ^ movie theaters (www.forbes.com)
- ^ Major League baseball games (www.sportstravelmagazine.com)
- ^ the upcoming school year (www.nature.com)
- ^ delta variant (theconversation.com)
- ^ responses to vaccines (doi.org)
- ^ specialist in pediatric infectious diseases (www.pediatrics.pitt.edu)
- ^ Pittsburgh Vaccine Trials Unit (covid19research.pitt.edu)
- ^ COVID-19 Prevention Network (www.coronaviruspreventionnetwork.org)
- ^ Jeff Kowalsky/AFP via Getty Images (www.gettyimages.com)
- ^ 2 to 15 years (www.businessinsider.com)
- ^ Operation Warp Speed (doi.org)
- ^ began receiving (www.washingtonpost.com)
- ^ several phases of clinical trials (theconversation.com)
- ^ rigorous evaluation process (www.fda.gov)
- ^ tens (www.nejm.org)
- ^ of thousands (www.nejm.org)
- ^ of adults (www.statnews.com)
- ^ three vaccines (covid19.trackvaccines.org)
- ^ authorized for emergency use (www.fda.gov)
- ^ brains are developing rapidly (doi.org)
- ^ antibodies they received from their mothers (doi.org)
- ^ Pneumococcal vaccine that prevents infections (www.cdc.gov)
- ^ unique version of the vaccine (www.chop.edu)
- ^ NurPhoto/Getty Images (www.gettyimages.com)
- ^ Sign up for The Conversation’s daily newsletter (theconversation.com)
- ^ vaccines for children under 12 (www.nbcnews.com)

















